A paper by Meyer et al. (2020) has a nice summary of potential mechanisms through which the presence of COVID-19 could impact clinical trial results.
What should a researcher do when a clinical trial is impacted by COVID-19? Here are some analysis considerations.
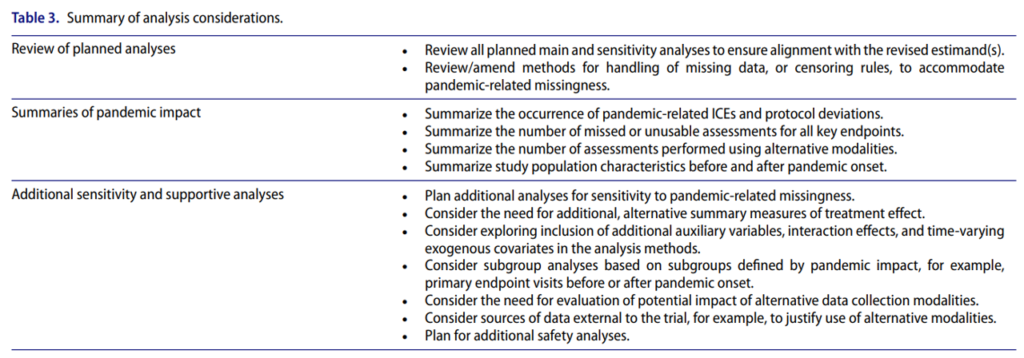
The full article is interesting throughout.










